Performance for
OA knee pain reduction
that matters


After 3 injections,
patients had
significantly less
pain associated with
5 basic functions
The effectiveness and safety of EUFLEXXA was shown to be comparable to another HA product in a head-to-head clinical trial. The study was conducted in 321 patients with
OA knee pain over a 12-week period.*
Pain improvement with EUFLEXXA was 62% (P<0.0001) vs 55% with the other product (P<0.0001).†
Most common adverse events include joint pain (11/160), increase in blood pressure (3/160), joint swelling (3/160), feeling of sickness (3/160), tingling (2/160), back pain (1/160), nausea (1/160), skin irritation (1/160).

HA=hyaluronic acid; OA=osteoarthritis.
*Questions based on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). WOMAC is an internationally recognized and widely used set of standardized questionnaires that allow healthcare providers to assess the condition of patients with osteoarthritis of the knee and hip. Measures include pain, stiffness, and physical functioning of the joints.
†Improvements from baseline were statistically significant for both treatment groups. EUFLEXXA = (101/160)
‡ Based on % reduction in WOMAC pain score from baseline in a pivotal, 12-week trial of EUFLEXXA (n=157) vs Synvisc (n=158). Pain-free defined as symptom-free for the 5 WOMAC pain questions (with average visual analog scale [VAS] scores of <20 mm).
In a clinical trial, significantly fewer unilateral*
patients treated
with EUFLEXXA
had a need for acetaminophen vs another
HA (P=0.001).
HA=hyaluronic acid; OA=osteoarthritis.
Acetaminophen was the only additional medication provided.
*Unilateral means OA knee pain in one knee.
†EUFLEXXA 51% (34 of 67 patients with unilateral knee pain)
another HA 18% (13 of 72 patients with unilateral knee pain).
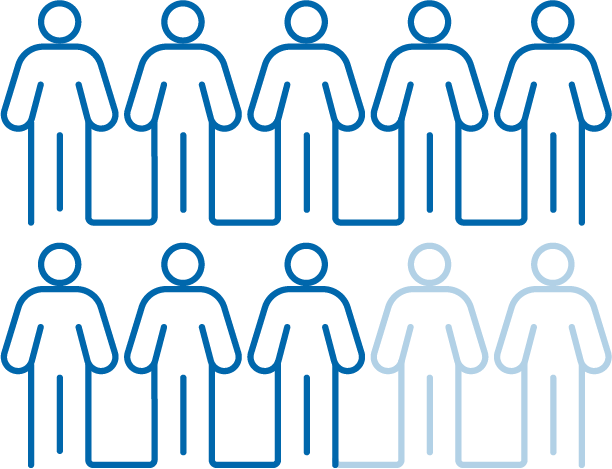

OA=osteoarthritis.
*In the 12-week study, side effects caused by EUFLEXXA were
joint pain (11/160), increase in blood pressure
(3/160), joint
swelling (3/160), feeling of sickness (3/160), tingling (2/160),
back pain (1/160), nausea (1/160), skin irritation (1/160), and tenderness in study knee (1/160).
1. #1 prescribed HA is based on rolling 12-month average of IQVIA claims data from unique patients (November 2021).
2. Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the
treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(2):154-162.
2. Data on file. Ferring Pharmaceuticals Inc.
EUFLEXXA (1% sodium hyaluronate) is used to relieve knee pain due to osteoarthritis. It is used for patients who do not get enough relief from simple pain medications such as acetaminophen or from exercise and physical therapy.
EUFLEXXA is only for injection into the knee, performed by a doctor or other qualified healthcare professional.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.FDA.gov/medwatch or call 1-800-FDA-1088.
You may also contact Ferring Pharmaceuticals Inc. at 1-888-FERRING.
 Video Play button
Video Play button

Find a healthcare provider near you who treats knee pain with EUFLEXXA®.
Find a Doctor >When dog trainer Jan first experienced knee pain, she tried treating it on her own for some time, but the pain became too much for her to bear. Learn how she managed this debilitating condition with the help of her doctor and EUFLEXXA®. Click to play the video and find out if EUFLEXXA® may be right for you!









